Study finds one shot of HPV vaccine just as effective as two; cervical cancer screening guidelines updated
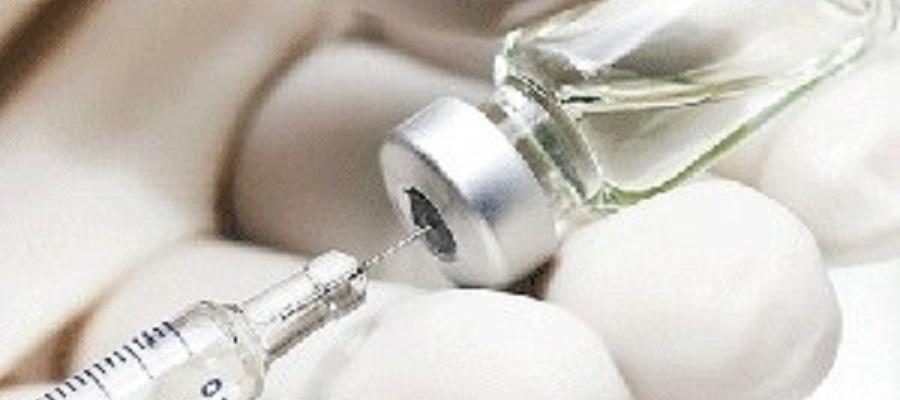
A study published Dec. 3 by the New England Journal of Medicine found that one dose of the HPV vaccine could be as effective as two in preventing cervical cancer. The study, led by the National Cancer Institute, consisted of a randomized trial of 20,320 girls ages 12-16. In addition, the American Cancer Society Dec. 4 announced updated guidelines for cervical cancer screening, saying that self-collection of vaginal samples for HPV testing is now optional, although clinician-collected samples are preferred.
Related News Articles
Headline
A JAMA study released Jan. 22 found that colorectal cancer is the leading cause of cancer deaths in people under age 50. The study examined cancer mortality in…
Headline
The five-year survival rate for all cancers in the U.S. has reached 70% for the first time, according to a report published Jan. 13 by the American Cancer…
Headline
A study released Jan. 12 by the Journal of the American College of Cardiology analyzed the current state of heart health in the U.S., highlighting the…
Headline
The Health Resources and Services Administration yesterday announced updated cervical cancer screening guidelines, including optional self-collection of…
Headline
The Centers for Medicare & Medicaid Services Dec. 11 announced the launch of the Make America Healthy Again: Enhancing Lifestyle and Evaluating Value-based…
Headline
The Centers for Medicare & Medicaid Services Innovation Center will launch a new, outcome-aligned payment model for providers offering technology-supported…

